Artificial Forest - The One Great Step towards a Greener future
- Minseo Kim
- Aug 3, 2020
- 8 min read
By: Arundhati Surendhran, Minseo Kim, Nyah Lawrence, Tanvi Singh and Yomna Mohamed
This paper is an evaluation and proposal for the implementation of artificial photosynthesis in the future. The paper speaks of several methods to incorporate as many circumstantial variations as possible, but undoubtedly, several situations will differ from the ideal that we base our model on. However, from the research conducted, this paper suggests the model that the authors believe to be the ideal.
To begin with, it is important to note that our top priority from this artificial forest is to minimize the emission of Carbon Dioxide and add up the oxygen supply into the air, just like what trees do all the time. Considering the fact that the rate of emission of carbon dioxide from factories, vehicles and other sorts is incessantly accelerating, we seem to find an importance to take action to that.
The trees that undergo photosynthesis, converting carbon dioxide in the air into oxygen, are not only carbon neutral but are also very beneficial to the biosphere. However, it has a severe limitation as it takes a tremendously long time to grow them, and an astronomical sum of capital is needed to grow and preserve them.
From this perspective, this artificial forest system - mimicking the natural process of plants and trees undergoing photosynthesis - could save immense costs and time as all it requires is time to construct and a little bit of management after that
General Process

The artificial forest system would take up the space of around 600m^2 (60m X 10m). This is to arrange the individual trees in a linear line to maximize its exposure to sunlight. Also, the system would be covered in biopolymers around the system just like a greenhouse to prevent leakage of carbon dioxide in the initiation process.
There would be few storage tanks in the basement where it would be connected by the pipelines to the ground so that the conversion can take place from the carbon dioxide released.
Under government regulation, all the factories that are involved in producing carbon dioxide during their production process would be obliged to install this carbon dioxide store tank (using carbon adsorption technique which will be introduced later). Once the carbon dioxides are collected, they won’t be released into the air, instead, would be delivered to the artificial forest system so that the conversion can take place here.
The CO2 storage tanks will be placed in the basement and will be replaced with another one once the conversion is complete. During the conversion, this gas would be released slowly in the forest system so that the reaction can take place on the leaves and the trunks, the photocatalysts. Meanwhile, the water-splitting process would take place in the basement where water will be split into Hydrogen (H2) and Oxygen (O2). H2 would be expected to travel through the pipeline while the oxygen would be released directly into the air. The splitting will be conducted by the cell which is used in fuel cell electric vehicles (FCEV) which is very efficient in releasing hydrogen and oxygen from water.
On the other hand, on the leaves and the trunks, the CO2 will be reduced into carbon monoxide (CO) and Formic acids (HCOOH) which would travel down the pipeline and react with hydrogens so that useful fuels can be produced. This includes methanol, ethanol and propanol which can later be saved in the fuel tank in the basement. For instance, the leaves, which act as a photocatalyst, were discovered by the researchers in Tokyo Institute of Science and Technology. They are very economical catalysts made up of abundant matters like copper and manganese which have a high-efficiency conversion rate of 57%.
Carbon Capture
Carbon capture is an essential process for the effectiveness of artificial photosynthesis, as a constant supply of the gas allows for greater amounts of photosynthetic activity. The following video talks about using adsorption to capture CO2 from the air as we deem it as the most efficient and suitable method in our case.
A post-graduate researcher at MIT developed a CO2 capture battery which uses electricity and adsorption to capture CO2 from the air regardless of its concentration in the feed gas, which in our case is air.
If there is a successful intervention by the government to oblige factories to install this carbon capturing batteries, it will certainly have immediate impact in reducing the emission of Carbon Dioxide gas in the air.
Leaves
Though this paper means to present the most ideal situation, it would not be pertinent to ignore the fact that each nation has different capabilities and desired outcomes when it comes to artificial photosynthesis, and there is also the fact that this may evolve into a privatised business in the future. In accordance, this paper will be illustrating all the different types of artificial leaves that have hitherto come forth.
Primarily, we would have to establish the duty of the artificial leaf, and how they will be mimicking their natural counterparts. The most primary thing is the splitting of water to form hydrogen and oxygen using sunlight, photolysis. To do this leaves separate the two half-reactions that produce Hydrogen and Oxygen, with a membrane separating the two - so that they do not explode - which still allows ion exchange, an important factor in maintaining charge balances.
Artificial Systems would mimic this with two photo-electrodes submerged in water for both the half-reactions, two catalysts to speed up the reactions and a membrane to keep the Photoelectrochemical cell, or ‘PEC’ from combusting.

The first noteworthy model is the Lewis model, who’s vision looks more like a carpet than a tree, with drainage pipes connected to drain out the liquid hydrogen produced. The system only does photolysis and does not produce anything but hydrogen and oxygen, but the hydrogen can be either used as a fuel or as feedstock for other heavier fuels such as gasoline.
Then we have the second model, from Dr Daniel Nocera, which unlike Lewis - who believes in producing Hydrogen as an intermediary - wishes to produce Carbon-based fuels directly through a biohybrid process. The initial process is much the same in regards to producing hydrogen using the inorganic catalyst that is a cobalt phosphorus alloy, but thereafter the hydrogen along with co2 is pumped into a pool of genetically modified bacteria which use these materials to produce carbon fuels, this has an efficiency of about 10%. The cobalt phosphate compound does not harm the bacteria, and is also self-assembled from the solution, imitating the ‘self-healing’ catalysts found in nature.
Moving further along, once again using a pool rather than an out-of-ground structure, Yimin Wu’s model is also as far from a ‘leaf’ as something can get. The model works based on pumping glucose, copper acetate, sodium dodecyl sulfate and sodium hydroxide into a pool. Then the catalyst ( a cheap powder - cuprous oxide) is added along with co2, with a beam of sunlight being focused on the system. The reaction produces oxygen, while also converting co2 into methanol in the solution, the methanol being collected as it evaporates.
From the University of Oslo, Dr Xu and their team believe that CO2 can be converted into formic acid through the use of an enzyme and a catalyst in the presence of sunlight - and they are correct! Tantalum Nitride is the perfect catalyst for this process, and it pushes the enzyme, “oxygen-tolerant formate dehydrogenase” according to Xu, to turn CO2 into formic acid.
The Yang model, rather than forming a fuel, forms a useful component that can be further used in industrial processes. The model is a nanowire-bacteria hybrid system - beginning with energy provided to bacteria using the nanowires, after which the Genetically Modified bacteria will reduce the CO2 into acetate ions, which is the most used base material in biosynthesis.
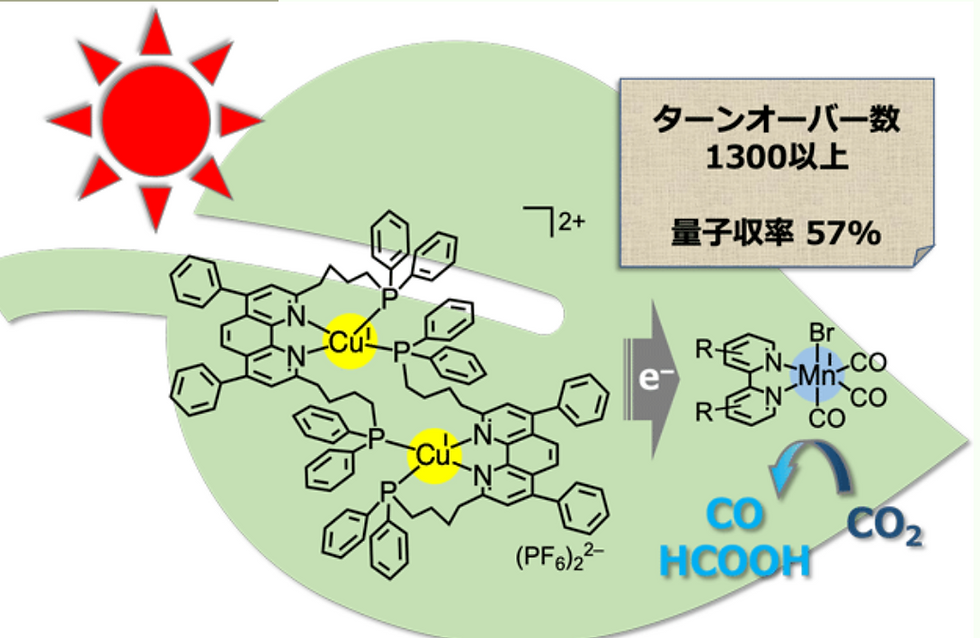
The final model this paper will discuss is one presented by the Japanese Institute of Technology, stating that using copper and manganese complexes as catalysts, one can reduce CO₂ into CO and HCOOH, both of which can be further used as industrial base materials for useful production.
Evidently, there are an array of models for the artificial leaf, and many more on the way, hence we can joyfully predict a future where easily made fuels are abundant rather than one of destruction due to the presence of a slow-working, yet deadly gas.
Artificial Silicon Trunk
In a model created by UC Berkeley researcher Peidong Yang, Artificial trees included nanowires heterostructures made of silicon trunks and titanium dioxide branches. A Z-scheme is used, similar to ones used by plants, where electrons are removed from the water and move through multiple molecules until it eventually is used to convert carbon dioxide into carbohydrates. Instead of natural molecules, the electron moves through silicon and titanium dioxide in the artificial tree.
Yang’s model uses photogenerated electrons in the silicon trunk to reduce hydrogen which is used for further reactions, however, in our model, rather than hydrogen ions being reduced, liquid hydrogen is supplied to the trunk by internal pipes from an underground hydrogen tank. Silicon is used as the trunk material because of its photocatalytic ability under visible light.
Bacteria present on the surface of the silicon nanowires then use this hydrogen to reduce carbon dioxide - which has been released into the contained artificial forest from the underground carbon dioxide storage containers - to produce biofuels or other chemical products such as acetate.
This model is extremely efficient as both the branches (or leaves) and the trunk of the “tree” produces usable carbon biofuels. This has many obvious benefits for the environment and could be a major step towards a more sustainable future.
Larger Overview
The benefits of biomimetic trees are those of a significant nature, as they have to do with the aid and support of our environment and ecosystem. Like any other tree, these artificial trees have the main purpose of providing oxygen to the atmosphere and removing carbon dioxide from its surroundings. However, unlike natural afforestation, the costs of maintaining these trees will be much less, and will also have an added benefit, due to its biopolymer structure. These trees are a sustainable source of oxygen, and will hopefully be a major contributor to the eradication of excess greenhouse gases. As mentioned previously, these trees are made from biopolymers, which serve as a multipurpose tool, since they have the potential to generate hydrogen-based fuels due to the artificial photosynthesis process. A striking characteristic of these trees is that they look modern, and will be easily integrated into the progressive architecture.
Even though the benefits of these trees heavily outweigh its drawbacks, it still has a few. As these trees are not common in the environment as of now, there may be difficulty in its implementation due to government and economic restrictions. There also may be a lack of space and safe land to build the trees on, in places that are prone to natural disaster or have barren ground.
As of 2018, 22% of greenhouse gas emissions come from industrial areas, due to the burning of fossil fuels. To ensure that the oxygen levels in industrial areas are being replenished often, artificial methods are deemed necessary. As a result of what was previously mentioned, the biomimetic trees will need to be implemented in areas like this.
Underground carbon dioxide storage will be what makes the artificial trees useful, since collected carbon from all over the region will be catalysed, and turned into useful fuel by the artificial photosynthesis process. The transportation of carbon dioxide from the storage will be done by pipeline, which will successfully transport tonnes of carbon dioxide to the artificial forest.
Higher oxygen levels in the atmosphere as a result of being a waste product of photosynthesis, leading to the slowing down of the rise in polar temperatures. When oxygen levels are higher, the atmosphere is not as thin, and so less water vapour to trap.
Reference sites: https://sp.lyellcollection.org/content/233/1/181





Comments